A brief timeline on the use of Vaxzevria (AstraZeneca) in Italy:
- On 29 January 2021, the European Medicines Agency (EMA) approved the use of Vaxzevria.
- The Italian Medicines Agency (Agenzia Italiana del Farmaco – AIFA) authorized the vaccine in Italy on 30 January 2021, and recommended that the latter be used only for people between the age of 18 and 55. Initially, Vaxzevria was used to vaccinate armed and police forces, school and university personnel, prison staff, and prisoners.
- On 22 February 2021, the Minister for Healthcare issued an internal communication increasing the age limit from 55 to 65. On 8 March 2021, he authorized the use of the vaccine for people over the age of 65.
- In mid-March 2021, after the first suspect cases of blood clots disorders (especially for women between the age of 25 and 65), Italy decided to suspend, on a precautionary basis, the inoculation of this vaccine.
- On 18 March 2021, the EMA issued a press release confirming that the vaccine's benefits continued to outweigh the risk of side effects, and that the vaccine was not associated with an increase in the overall risk of blood clots for those who received it. Italy consequently decided to resume vaccination with Vaxzevria.
- Other European countries and Canada decided to suspend Vaxzevria on 31 March 2021, but EMA reiterated that no particular blood clot risk factors were linked to this vaccine.
- However, through an internal communication, the Ministry for Healthcare recommended on 7 April 2021 that Vaxzevria be used for individuals over the age of 60. Both EMA and AIFA highlighted that the risk-benefit ratio was still in favour of the vaccine, confirming that the thrombosis cases that were being registered were associated with low platelet counts and should be listed as infrequent side effects, specifically hitting women under the age of 60.
- On 10 June 2021, AIFA released its fifth report on the surveillance of Covid-19 vaccines. It stated that, for people falling in the under-60 age group, there had been one thrombosis case every 100,000 people who received the first dose of Vaxzevria. There were no cases after the second dose.
- On 11 June 2021, the Pharmacovigilance Risk Assessment Committee (PRAC) of the EMA established that people with a previous medical history of capillary leak syndrome could not be vaccinated with Vaxzevria.
- After AIFA’s recommendation, and through an internal communication dated 14 June 2021, the Ministry for Healthcare determined that the vaccine would be used for people above the age of 60. For those under the age of 60 who received the first dose, the cycle would be completed with a second dose of either Comirnaty or Moderna ('heterologous vaccination').
- On 18 June 2021, another internal communication was released, authorizing the completion of the vaccination cycle with Vaxzevria for those under the age of 60 who had received the first dose of Vaxzevria, but refused to complete the vaccination cycle with another vaccine. The second dose of Vaxzevria would be administered in these extraordinary cases only and strictly after the individual’s informed consent.
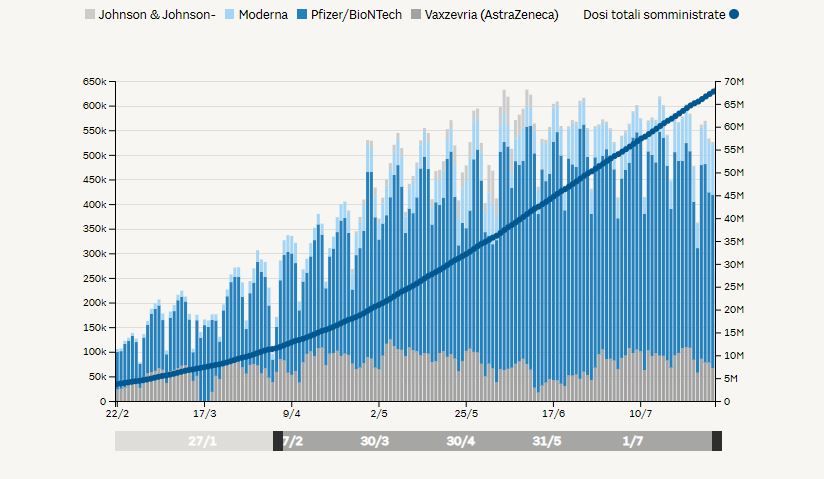
If we look at the available data above, we observe that the average inoculation of Vaxzevria was more or less constant since the vaccination campaign picked up towards the end of February 2021. The graph also portrays the moment when, mid-March, the use of Vaxzevria was suspended on a precautionary basis. However, data shows that, once the vaccine was re-introduced, its inoculation picked up rapidly. For what concerns the most recent episodes in the first-half of June 2021, we may notice a short decline which did not, however, affect the overall average use of Vaxzevria.
At first sight, it looks like the Ministry for Healthcare used scientific data to guide its decisions, aligning the latter with the recommendations received from both the EMA and AIFA, for the better part of the vaccination campaign. As the first thrombosis cases spread fear and distrust among the population, however, the executive showed signs of uncertainty, which surfaced in a long series of scattered, rash, and contradictory decisions.
The Vaxzevria 'affair' raises several questions. The most pressing issue, though, regards the principles that underpin the Italian institutional design. The last few months have blatantly shown the undefined and fragile relationship between politiké and téchne (politics and skilled knowledge) – epitomized by the relationship between the EMA and national authorities. It urges us to beg the question about the appropriate role of science in a political decision-making process. This issue calls for an even more profound question about political responsibility: can political actors and bodies use science to avoid responsibility? If the answer is 'no', we need to explore ways of holding political bodies into account as the playing field changes.
TWEET